For acromegaly patients on injectable somatostatin receptor ligands (SRLs), treatment-related symptoms and side effects can impair quality of life.1,2 Yet some hesitate to share this information with their physicians.3
All study patients were bothered by the amount of time they continued to experience acromegaly symptoms despite treatment.5
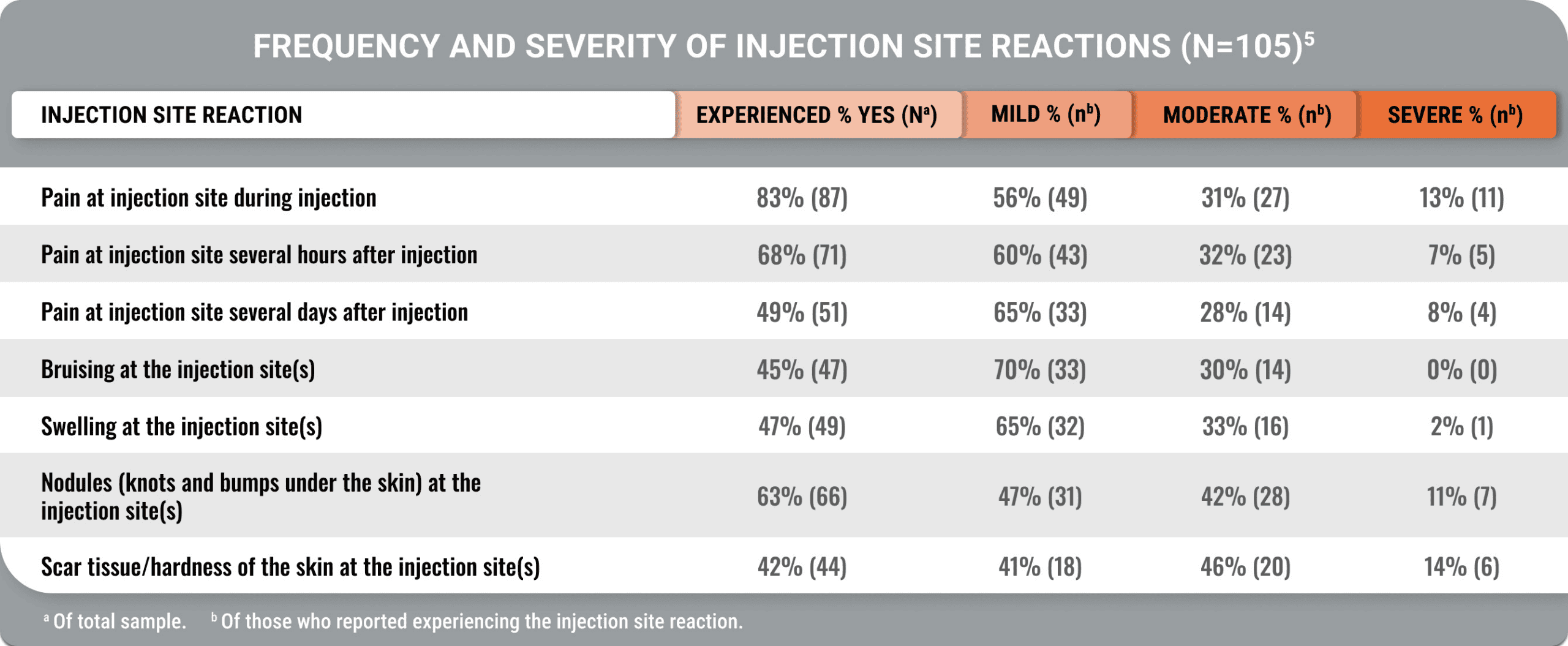
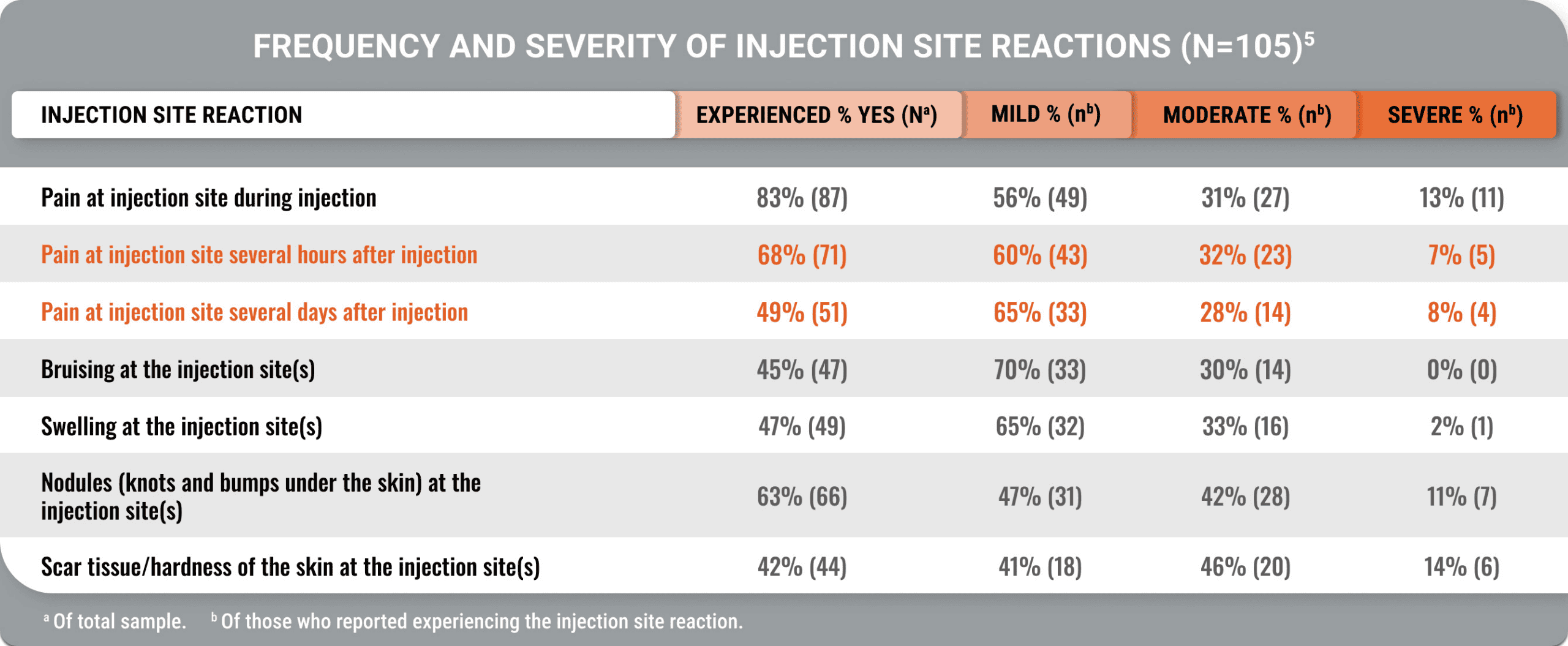
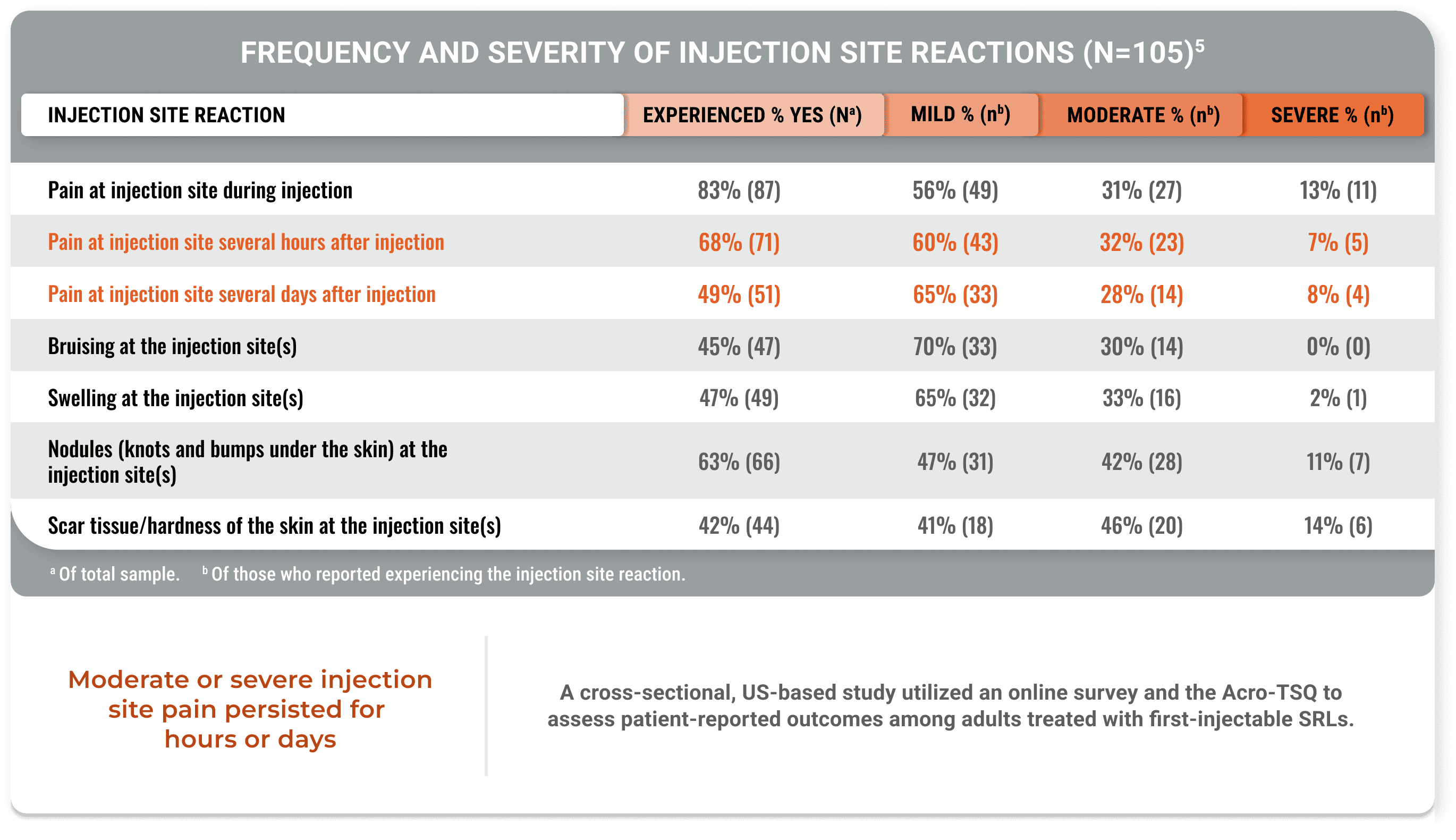
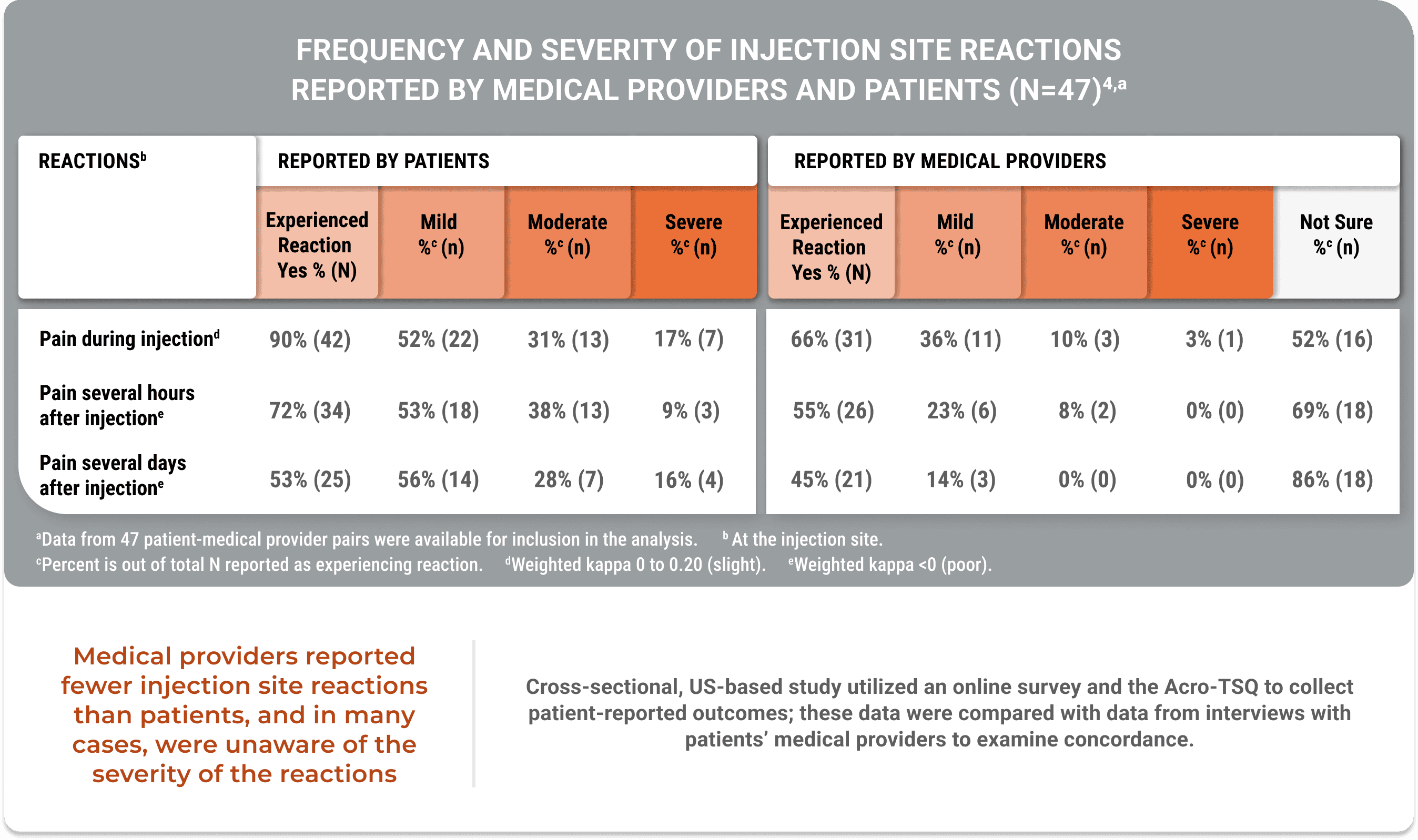
Lasting pain and other injection site reactions were typical in patients on a stable injectable SRL dose for ≥12 months (Geer et al).5
Moderate or severe injection site pain persisted for hours or days
A cross-sectional, US-based study utilized an online survey and the Acro-TSQ to assess patient-reported outcomes among adults treated with injectable SRLs.
View StudyThe Acromegaly Treatment Satisfaction Questionnaire (Acro-TSQ) follows FDA recommendations detailed in an official document guiding PRO development, which included qualitative research with individuals diagnosed with acromegaly.5
In an analysis of burdens associated with injectable SRLs (Fleseriu et al)1:
77%
57%

“It can’t be touched. The [injection] area, if it’s touched, it is extremely tender—exquisitely tender.” -Erin
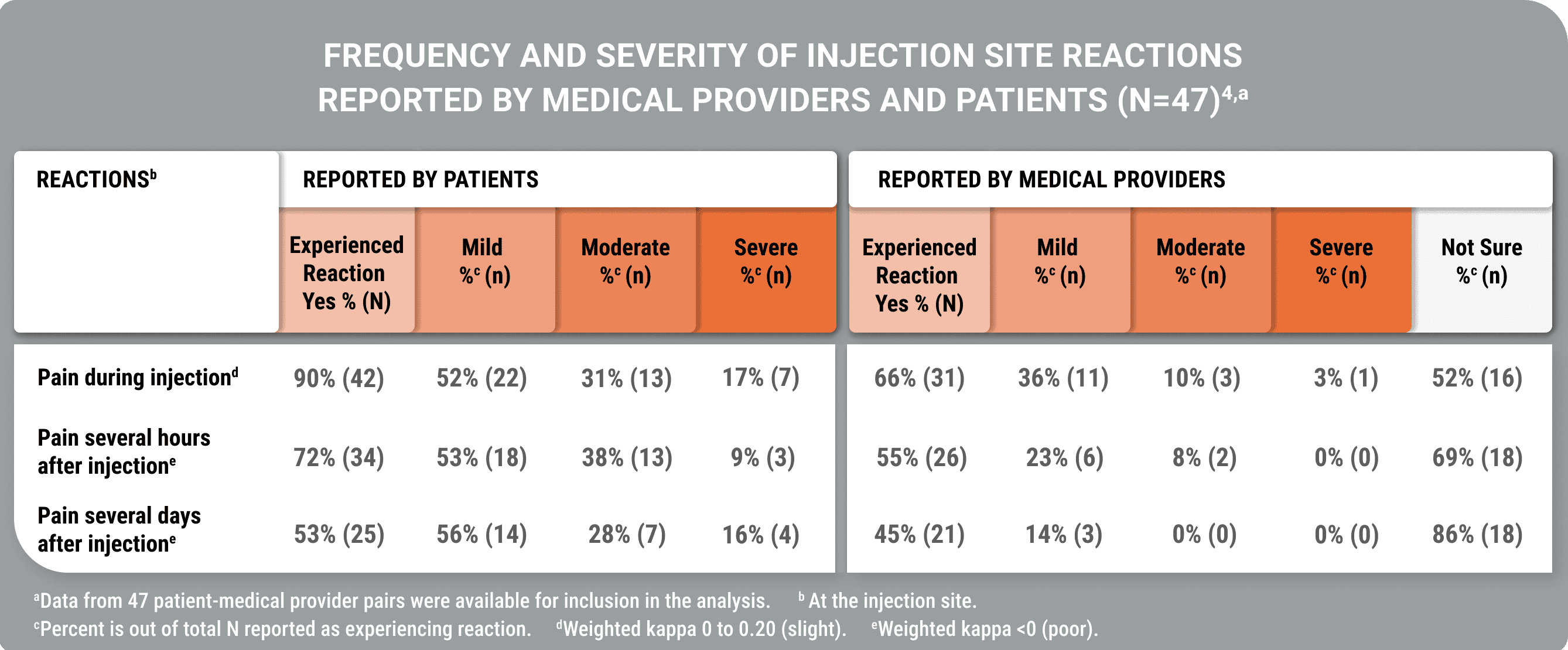
Poor agreement on injection site reaction severity was observed in a study analyzing concordance between patients and their physicians (Geer et al).4
Medical providers reported fewer injection site reactions than patients, and in many cases, were unaware of the severity of the reactions
A cross-sectional, US-based study utilized an online survey and the Acro-TSQ to collect patient-reported outcomes; these data were compared with data from interviews with patients’ medical providers to examine concordance.
View StudyThe Acromegaly Treatment Satisfaction Questionnaire (Acro-TSQ) follows FDA recommendations detailed in an official document guiding PRO development, which included qualitative research with individuals diagnosed with acromegaly.5
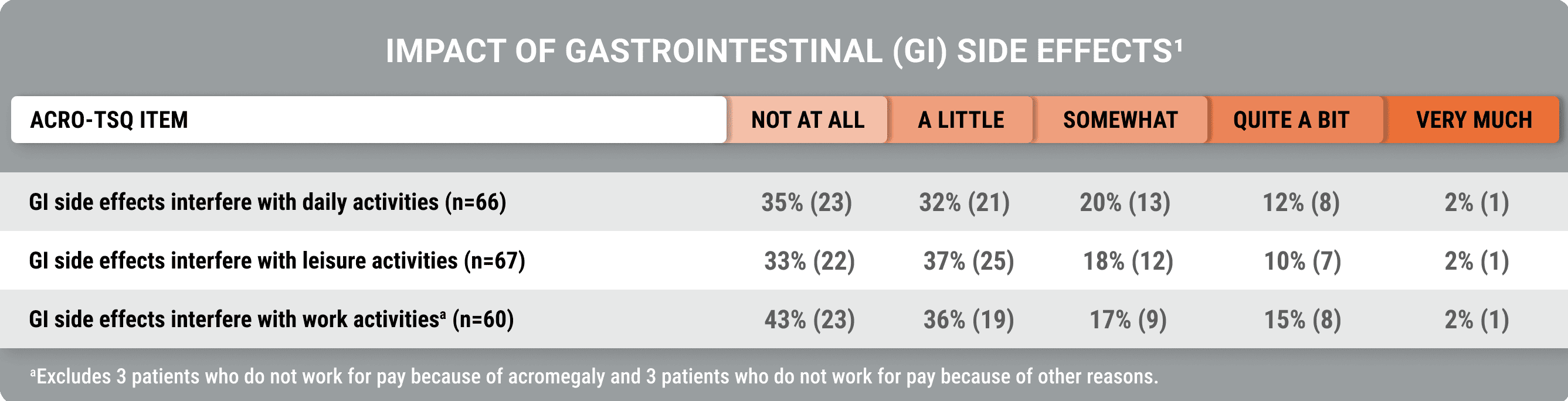
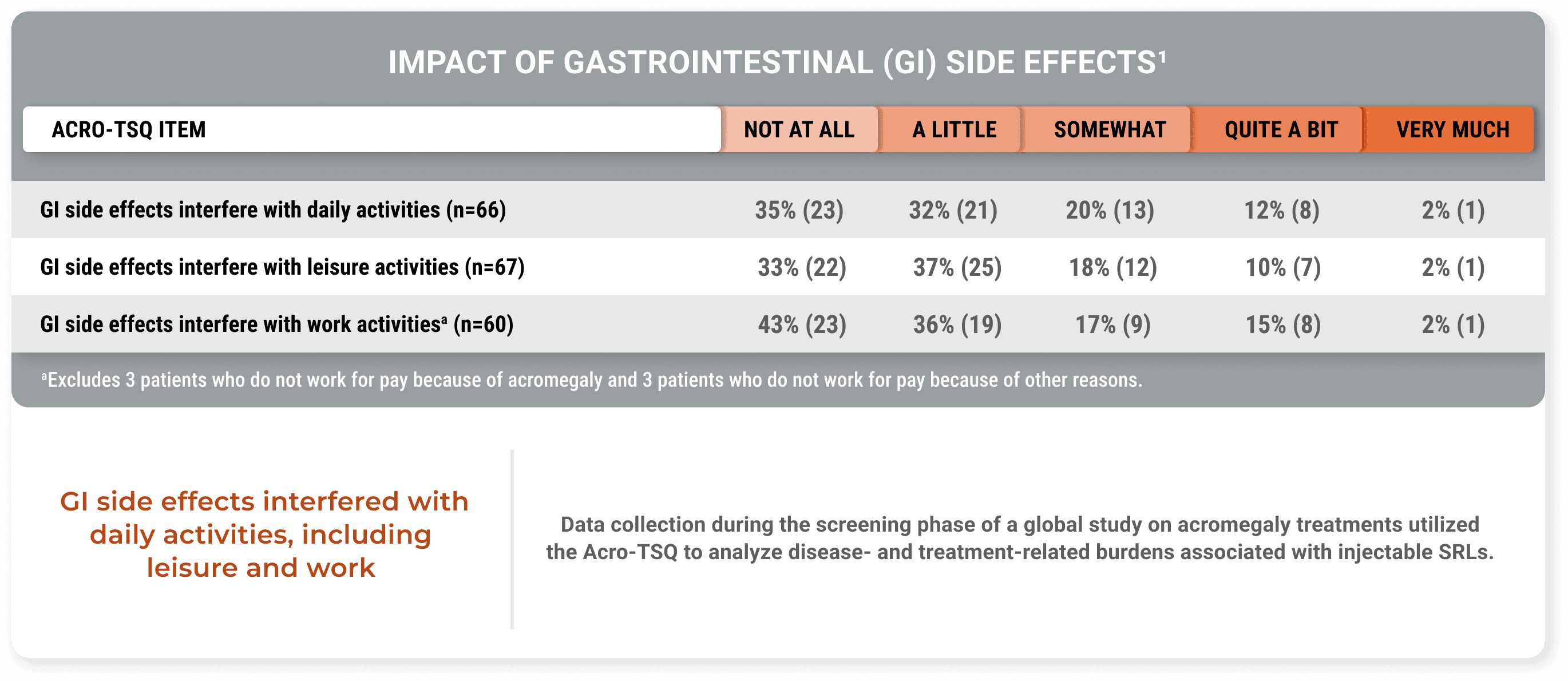
GI side effects impacted daily life in an analysis of burdens associated with injectable SRLs (Fleseriu et al).1
GI side effects interfered with daily activities, including leisure and work
Data collection during the screening phase of a global study on acromegaly treatments utilized the Acro-TSQ to analyze disease- and treatment-related burdens associated with injectable SRLs.
View StudyThe Acromegaly Treatment Satisfaction Questionnaire (Acro-TSQ) follows FDA recommendations detailed in an official document guiding PRO development, which included qualitative research with individuals diagnosed with acromegaly.5
In patients on a stable injectable SRL dose for ≥12 months (Geer et al)5:
72%


“I have real bad nausea, just violent vomiting.” -David
In an assessment of acromegaly disease burden, quality of life, and treatment satisfaction (Liu et al)2:
84%

“The breakthrough symptom that I feel is joint stiffness, especially in my hip flexors.” -Wendy
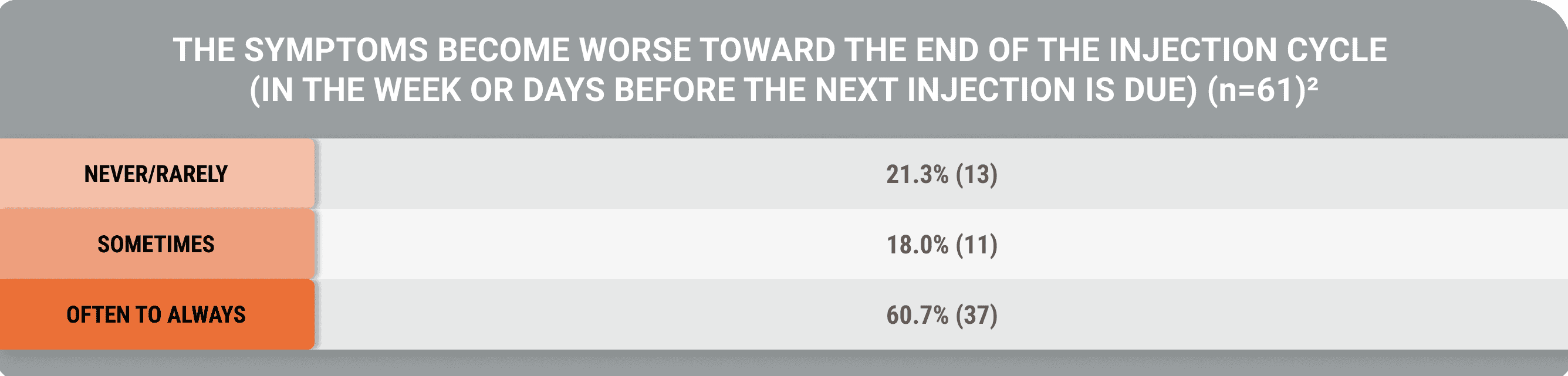
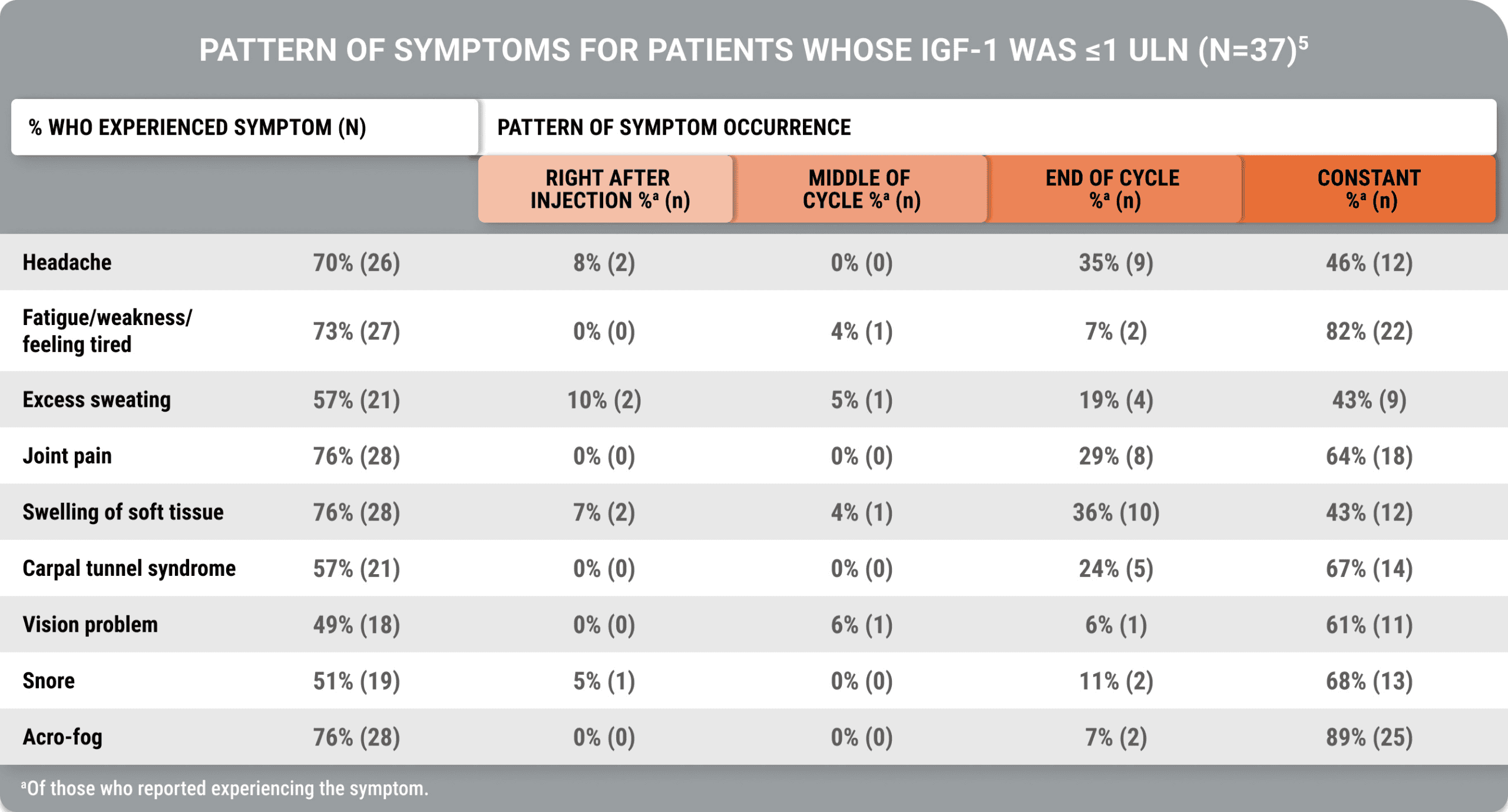
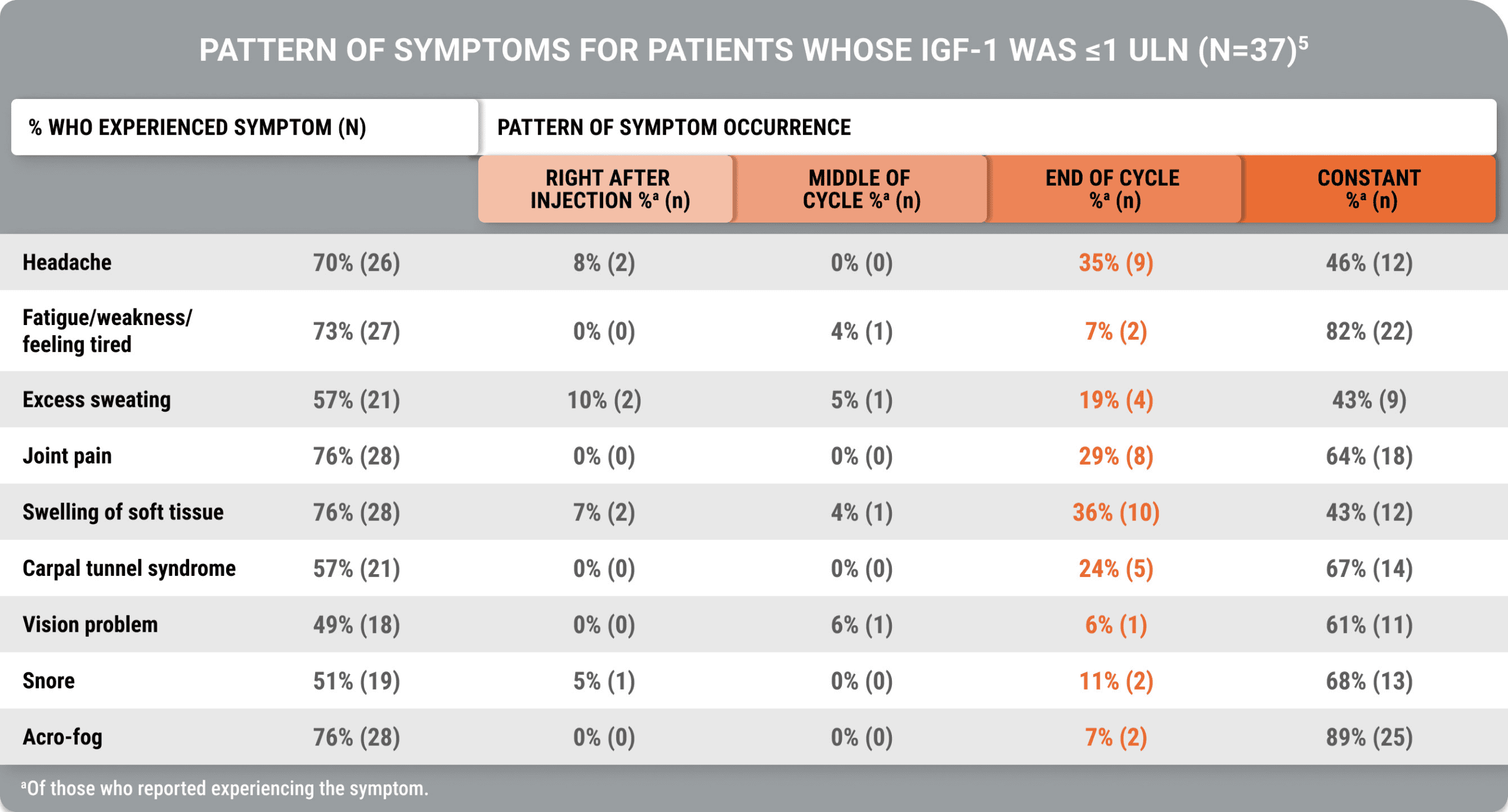
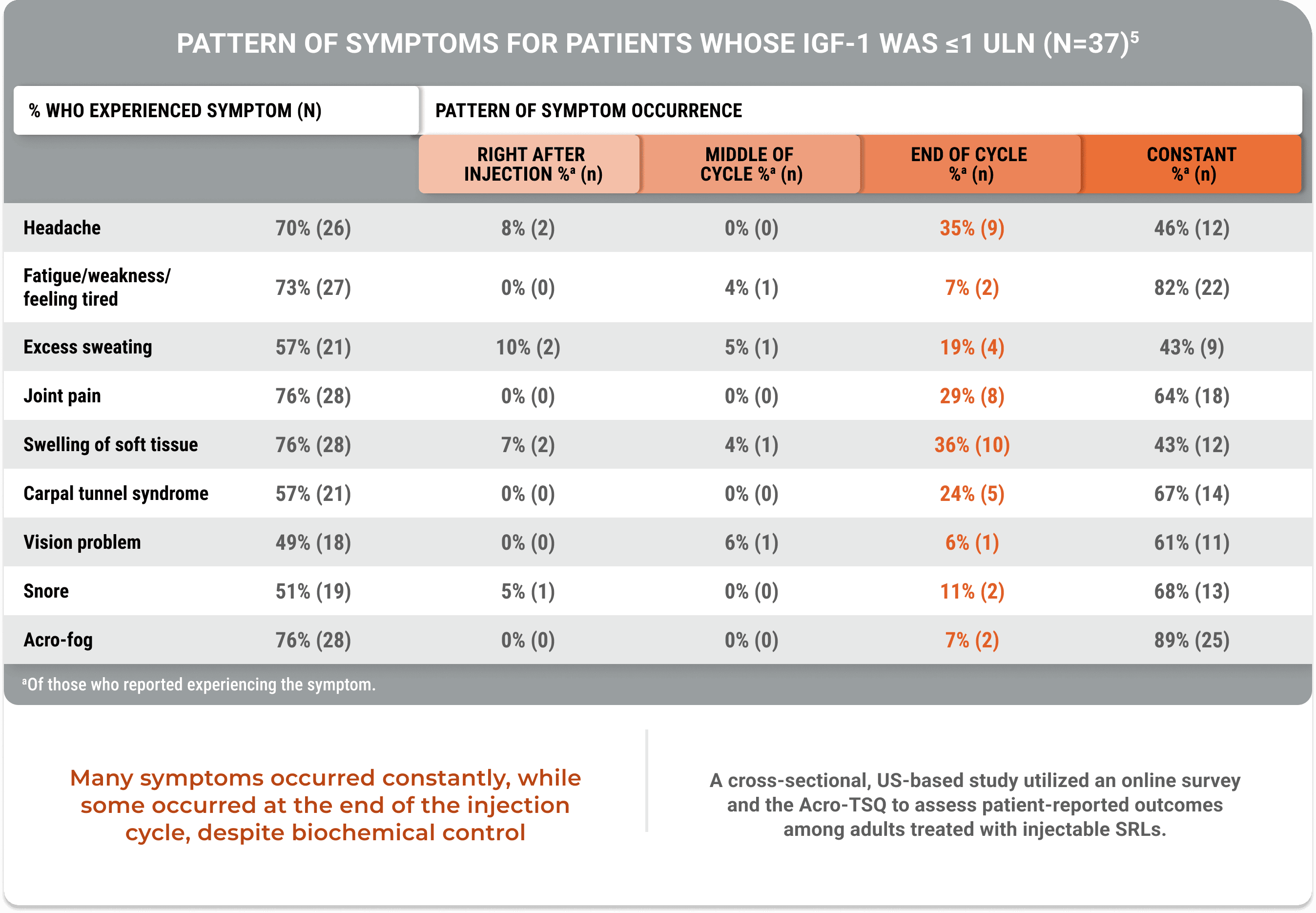
An additional study demonstrated that despite biochemical control, acromegaly symptoms occurred (Geer et al).5
Many symptoms occurred constantly, while some occurred at the end of the injection cycle, despite biochemical control
A cross-sectional, US-based study utilized an online survey and the Acro-TSQ to assess patient-reported outcomes among adults treated with injectable SRLs.
View StudyThe Acromegaly Treatment Satisfaction Questionnaire (Acro-TSQ) follows FDA recommendations detailed in an official document guiding PRO development, which included qualitative research with individuals diagnosed with acromegaly.5
In an analysis of burdens associated with injectable SRLs (Fleseriu et al)1:
64%

“So, my husband does my injection at home, and he feels guilty. He’s always worried that he’s hurting me.” -Ellen
In this study, many patients reported treatment-related sadness, treatment-related anxiety, and frustration with how they received treatment.1
Injectable SRL treatment is associated with additional ongoing challenges.1,6
Preparation/Administration
Issues such as clogged or broken needles often necessitated a second shot6
LOST WORK DAYS
Patients reported a mean of 11 lost work days per year6
treatment inconvenience
76% were bothered by having to travel for injections1
Gaining insights into patient challenges may help foster collaboration and increase patient satisfaction.3,a

Preparation/administration challenges

work
loss

Breakthrough
symptoms

Emotional
distress

“I’VE HAD A LOT OF MISHAPS…THE NEEDLE CLOGS SOMETIMES NO MATTER WHOSE HANDS IT’S IN.” -Ellen
“I was so extremely fatigued…I couldn’t do my job.” -Wendy
“Every 21 days or so, it’s a recurring monthly nightmare.” -David
“I DREADED GETTING THIS INJECTION…THE PAIN THAT WAS ACUTE, THE SORENESS I WOULD HAVE FOR DAYS.” -Erin
–
“I HAD TERRIBLE SCAR TISSUE DEVELOPMENT THAT HAPPENED. AND SO IT WAS EXTREMELY PAINFUL TO GET INJECTIONS.” -Erin
“I like to go out of town…but I have to be at home to get that injection.” -Wendy
“I have real bad nausea, violent vomiting for a couple of days after.” -David
“DRIVING TO AN INFUSION CENTER, HAVING A DIFFERENT NURSE EVERY TIME…WAS JUST SO STRESSFUL.” -Ellen

Injection site
reactions

Treatment
inconvenience

GI side
effects

DEPENDENCE
ON OTHERS
Consider discussing the specific burdens your patients may be facing on injectable SRLs.
References
- Fleseriu M, Molitch M, Dreval A, et al. Disease and treatment-related burden in patients with acromegaly who are biochemically controlled on injectable somatostatin receptor ligands. Front Endocrinol (Lausanne). 2021;12:627711. doi:10.3389/fendo.2021.627711
- Liu S, Adelman DT, Xu Y, et al. Patient-centered assessment on disease burden, quality of life, and treatment satisfaction associated with acromegaly. J Investig Med. 2018;66(3):653–660. doi:10.1136/jim-2017-000570
- Gurel MH, Bruening PR, Rhodes C, Lomax KG. Patient perspectives on the impact of acromegaly: results from individual and group interviews. Patient Prefer Adherence. 2014;8:53-62. doi:10.2147/PPA.S56740
- Geer EB, Sisco J, Adelman DT, et al. Observed discordance between outcomes reported by acromegaly patients and their treating endocrinology medical provider. Pituitary. 2020;23(2):140-148. doi:10.1007/s11102-019-01013-2
- Geer EB, Sisco J, Adelman DT, et al. Patient reported outcome data from acromegaly patients treated with injectable somatostatin receptor ligands (SRLs) in routine clinical practice. BMC Endocr Disord. 2020;20(1):117. doi:10.1186/s12902-020-00595-4
- Strasburger CJ, Karavitaki N, Störmann S, et al. Patient-reported outcomes of parenteral somatostatin analogue injections in 195 patients with acromegaly. Eur J Endocrinol. 2016;174(3):355-362. doi:10.1530/EJE-15-1042